In the intricate world of manufacturing, maintaining impeccable quality is a continuous endeavor. Despite the most rigorous processes and highly skilled teams, the reality is that non-conformances – instances where a product, process, or service doesn’t meet specified requirements – are an inevitable part of operations. When these deviations occur, they aren’t just minor inconveniences; they can lead to costly reworks, production delays, customer dissatisfaction, and even reputational damage if not handled correctly.
The key to effectively managing these situations lies not in hoping they won’t happen, but in having a robust system in place to identify, document, address, and prevent their recurrence. This is precisely where a well-designed non-conformance report comes into play. It acts as the backbone of your quality control system, providing a structured approach to capturing all the critical information needed to resolve issues systematically.
Having a standardized manufacturing non conformance report template streamlines this entire process, ensuring consistency across all reported incidents. It removes guesswork, makes data collection efficient, and facilitates clear communication among all stakeholders, from production floor staff to quality engineers and management. This uniformity is crucial for effective analysis and continuous improvement efforts.
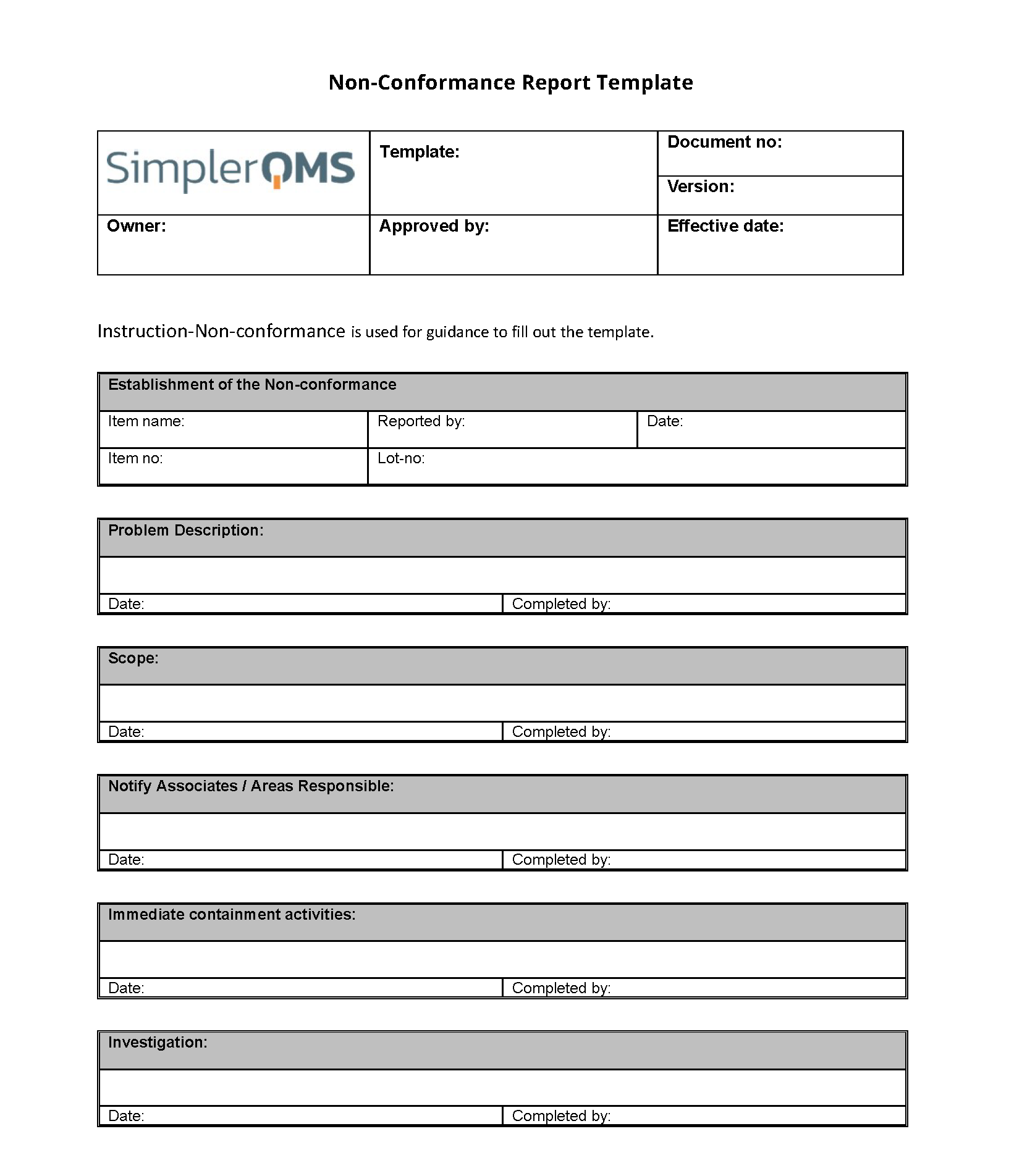
Understanding the Core Components of an Effective Non Conformance Report Template
A truly effective manufacturing non conformance report template isn’t just a simple form; it’s a comprehensive tool designed to guide you through the entire lifecycle of a quality issue, from its discovery to its ultimate resolution and prevention. It needs to capture granular details while remaining user-friendly, ensuring that every non-conformance, no matter how minor, receives the attention it deserves. Let’s delve into the essential sections that make up a robust template.
Key Information for Identification and Description
The first step in addressing any non-conformance is accurately identifying and describing it. Your template should start with fields that pinpoint exactly what went wrong, where, and when. This includes:
- Non-Conformance ID: A unique identifier for tracking purposes.
- Date and Time of Discovery: When the issue was first noticed.
- Location/Department: Where the non-conformance occurred (e.g., assembly line 3, raw material inspection).
- Product/Part Number and Description: Details of the affected item.
- Quantity Affected: How many units are involved.
- Description of Non-Conformance: A clear, objective explanation of the deviation from specifications. Include visual aids if possible.
- Reference Document(s): Which standard, drawing, or procedure was violated.
- Reported By: Name and department of the person who discovered the issue.
This initial data is vital for ensuring everyone understands the specifics of the problem at hand, setting the stage for effective investigation.
Once the non-conformance is identified, the next critical phase involves assessing its potential impact. This section helps determine the severity and urgency of the issue. It typically includes an initial assessment of the impact on safety, function, and schedule, as well as an immediate disposition recommendation, such as “hold,” “scrap,” or “rework.” This preliminary analysis guides immediate actions to contain the problem and prevent further complications.
Root Cause Analysis and Corrective Actions
Identifying the root cause is arguably the most crucial part of the non-conformance process. Without understanding why something went wrong, you’re merely treating symptoms. Your template needs dedicated sections for:
- Root Cause Investigation: Space to document the findings of the investigation, often using tools like the 5 Whys or Fishbone diagrams.
- Identified Root Cause(s): The fundamental reason(s) for the non-conformance.
- Corrective Action(s) Proposed: Specific steps to eliminate the identified root cause and prevent recurrence.
- Preventive Action(s) Proposed (if applicable): Steps to prevent similar non-conformances from happening anywhere else in the system.
- Responsibility and Due Date: Who is assigned to complete each action and by when.
These sections transform the report from a mere documentation tool into a powerful problem-solving instrument, driving true quality improvement. It’s not enough to fix the immediate problem; you must address the underlying issues to prevent a recurrence, ensuring long-term product integrity and process efficiency.
Finally, every good template includes provisions for verification and closure. This involves confirming that the corrective actions have been effectively implemented and that the non-conformance has been resolved. It should include fields for:
- Verification of Effectiveness: Details of how the corrective actions were checked and confirmed to be successful.
- Date of Closure: When the non-conformance report is officially closed.
- Closed By: Name of the person authorizing closure.
This final step ensures accountability and confirms that the quality system has learned from the incident, contributing to a cycle of continuous improvement.
Maximizing the Value of Your Non Conformance Reporting System
Implementing a robust manufacturing non conformance report template is just the first step; deriving maximum value from it requires consistent application, team training, and integration into your overall quality management system (QMS). Simply having a template isn’t enough; it’s how you use it that truly makes a difference in your manufacturing quality and operational efficiency.
One of the most critical aspects of maximizing its value is ensuring that all relevant personnel are thoroughly trained on how to use the template correctly. This includes not only quality control staff but also production operators, supervisors, and anyone else who might encounter or report a non-conformance. Clear guidelines on how to fill out each section, the importance of accurate data, and the subsequent steps in the non-conformance process will foster a culture of quality and accountability. Regular refresher training can help reinforce these practices and introduce any updates to the template or process.
Furthermore, integrating your non-conformance reports into a broader data analysis framework can unlock significant insights. By collecting data from multiple reports, you can identify recurring patterns, common root causes across different products or processes, and areas where your manufacturing operations might have systemic weaknesses. This data-driven approach allows you to move beyond reactive problem-solving to proactive prevention, leading to more substantial and sustainable improvements. Consider digitizing your template to enable easier data aggregation and trend analysis, potentially linking it with other QMS modules.
Adopting a well-structured manufacturing non conformance report template is more than just good practice; it’s a fundamental pillar of a thriving quality management system. By systematically documenting, investigating, and resolving quality deviations, manufacturers can transform potential weaknesses into opportunities for growth and refinement. This proactive approach not only enhances product quality and customer satisfaction but also fosters a culture of continuous improvement across the entire organization.
Embracing such a standardized tool empowers teams to identify problems swiftly, implement effective solutions, and prevent recurrence, ultimately strengthening operational integrity and ensuring long-term success in a competitive market. It’s an investment in efficiency, quality, and the reputation of your manufacturing enterprise.